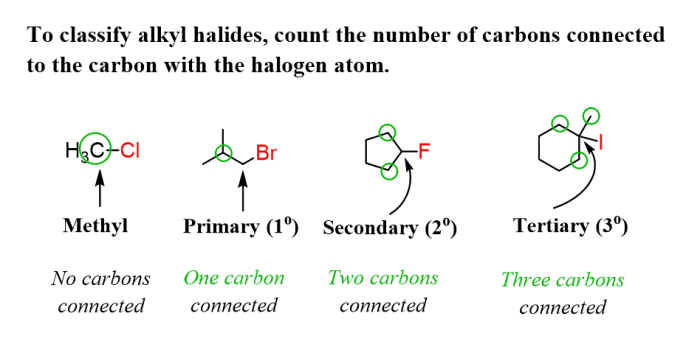
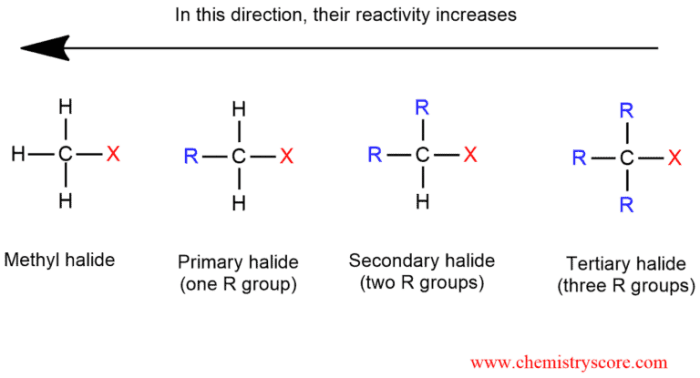
Rank the following compounds in order of decreasing sn1 reactivity – In this article, we will explore the concept of SN1 reactivity and rank a series of compounds based on their decreasing SN1 reactivity. SN1 reactivity is a measure of the rate at which a compound undergoes a substitution reaction via a unimolecular mechanism.
Understanding the factors that influence SN1 reactivity is crucial for predicting the reactivity of organic compounds and designing synthetic strategies.
SN1 Reactivity: Rank The Following Compounds In Order Of Decreasing Sn1 Reactivity
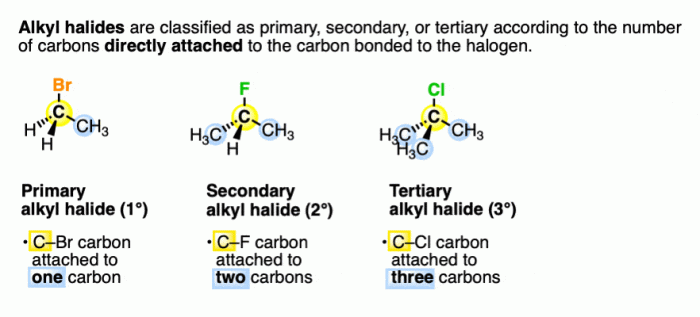
SN1 reactivity refers to the rate at which a unimolecular nucleophilic substitution (SN1) reaction occurs. SN1 reactions involve the substitution of a leaving group by a nucleophile in a single step, and the rate of the reaction is determined by the stability of the carbocation intermediate formed during the reaction.Factors
that affect SN1 reactivity include:
-
-*Leaving group ability
The leaving group ability of a group refers to its ability to depart from the substrate and form a stable anion. Good leaving groups are typically weak bases and can easily form stable anions.
-*Carbocation stability
The stability of the carbocation intermediate formed during the SN1 reaction is also important. More stable carbocations lead to faster SN1 reactions.
-*Solvent polarity
Polar solvents can help to stabilize the carbocation intermediate by solvating it. This can lead to faster SN1 reactions in polar solvents.
Compounds to be Ranked
The following compounds are to be ranked in order of decreasing SN1 reactivity:
- *CH3CH2Br
- *(CH3)2CHBr
- *(CH3)3CBr
- *CH3CH2Cl
- *(CH3)2CHCl
- *(CH3)3CCl
Ranking of Compounds
The compounds can be ranked in order of decreasing SN1 reactivity as follows:
- *(CH3)3CBr > (CH3)2CHBr > CH3CH2Br > (CH3)3CCl > (CH3)2CHCl > CH3CH2Cl
This ranking is based on the following factors:
-
-*Leaving group ability
Bromide is a better leaving group than chloride, so the compounds containing bromide will be more reactive in SN1 reactions.
-*Carbocation stability
The tertiary carbocation formed in the reaction of (CH3)3CBr is more stable than the secondary carbocation formed in the reaction of (CH3)2CHBr, which is more stable than the primary carbocation formed in the reaction of CH3CH2Br. This is because the more alkyl groups that are attached to the carbocation, the more stable it will be.
-*Solvent polarity
The ranking of the compounds will also depend on the polarity of the solvent. In polar solvents, the carbocation intermediate will be more stabilized, leading to faster SN1 reactions.
Discussion, Rank the following compounds in order of decreasing sn1 reactivity
The ranking of the compounds in order of decreasing SN1 reactivity can be used to predict the reactivity of other compounds in SN1 reactions. For example, if a compound contains a tertiary alkyl halide, it will be more reactive in SN1 reactions than a compound that contains a secondary alkyl halide, which will be more reactive than a compound that contains a primary alkyl halide.The
ranking of the compounds can also be used to design synthetic strategies. For example, if a chemist wants to carry out a SN1 reaction on a substrate that contains a primary alkyl halide, they may need to use a more polar solvent or a stronger nucleophile to achieve a good yield of product.
FAQ Corner
What is SN1 reactivity?
SN1 reactivity refers to the rate at which a compound undergoes a substitution reaction via a unimolecular mechanism, involving the formation of a carbocation intermediate.
What factors affect SN1 reactivity?
SN1 reactivity is influenced by several factors, including the stability of the carbocation intermediate, the nature of the leaving group, and the polarity of the solvent.